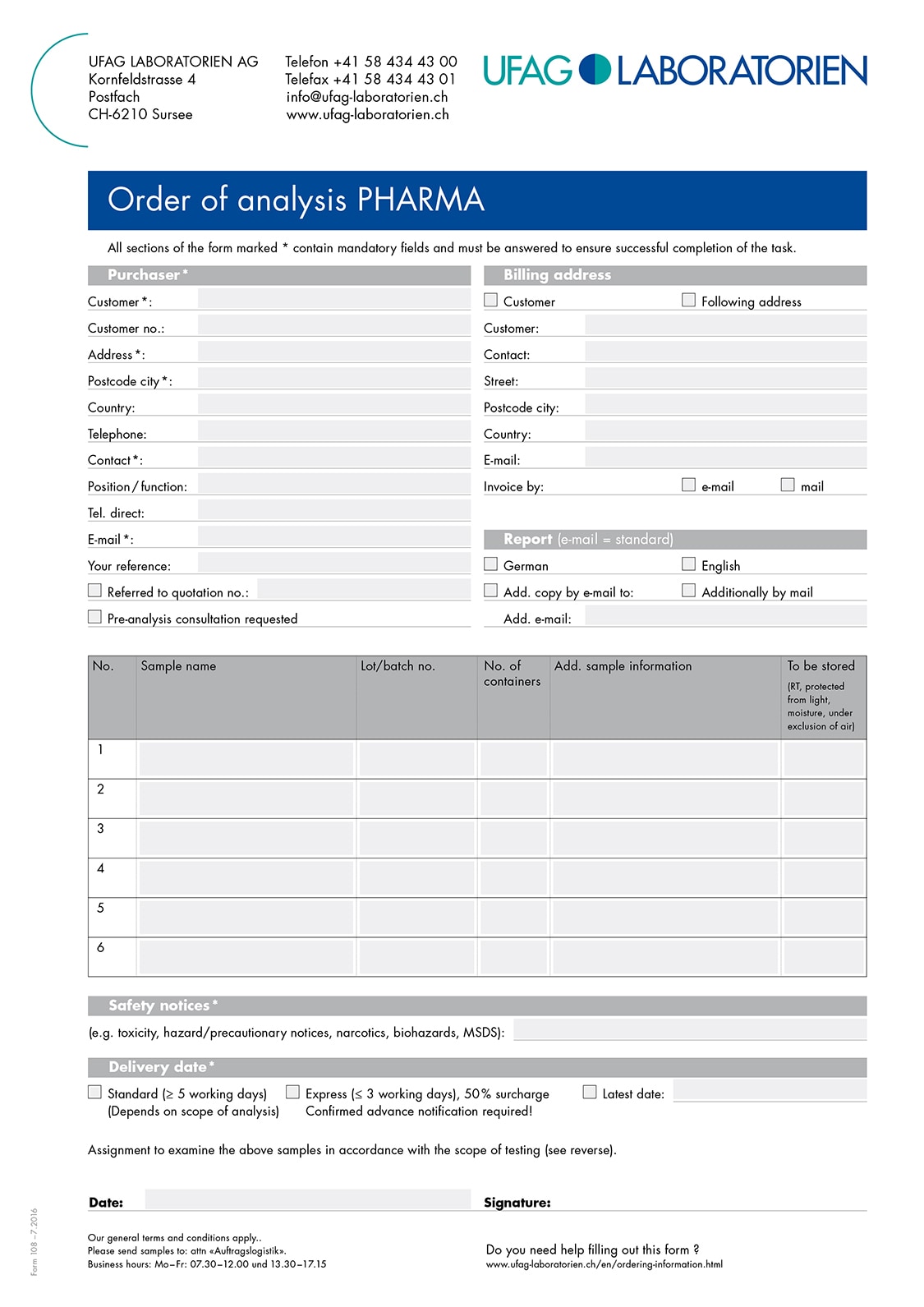
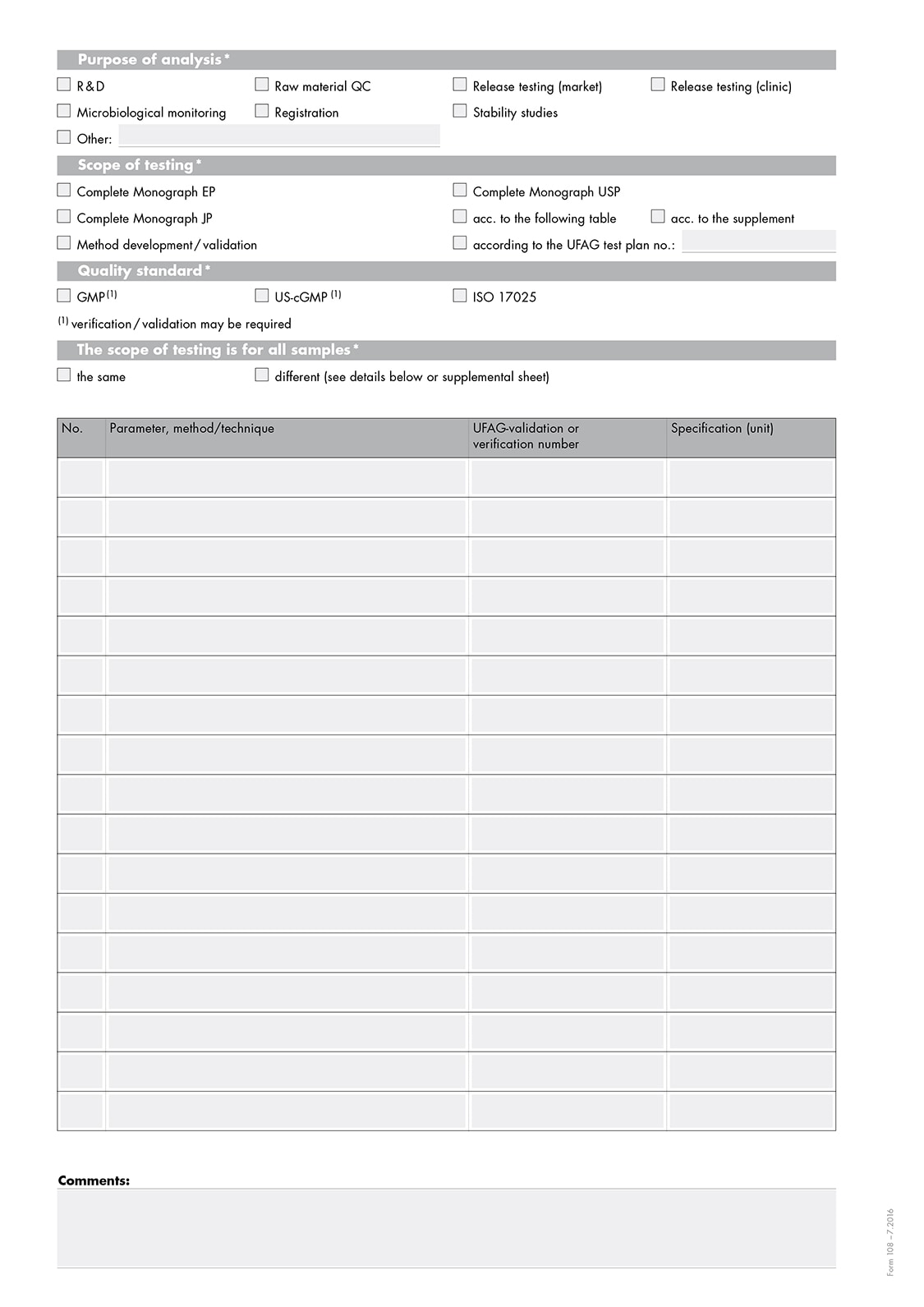
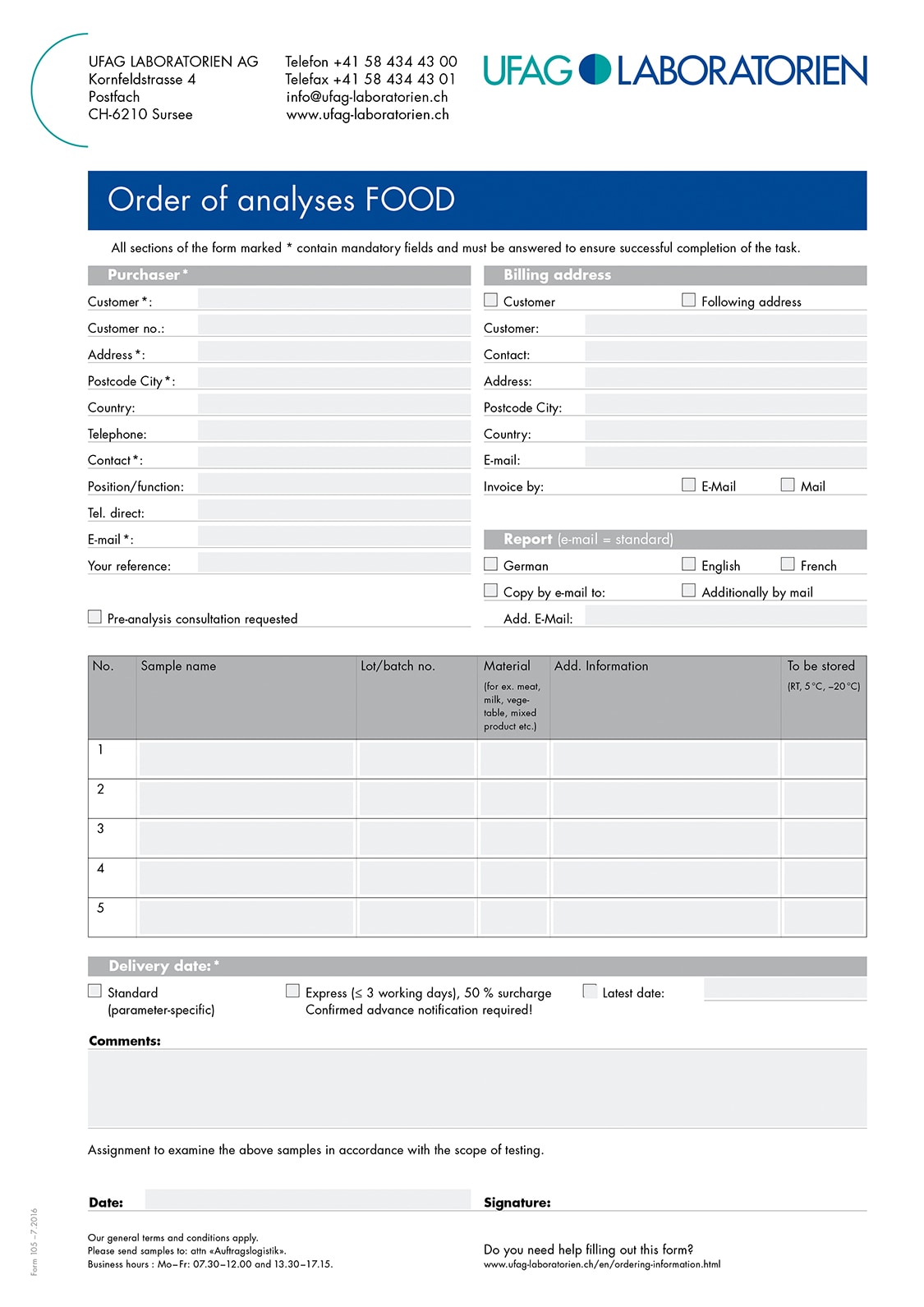
Sending samples from the EU to Switzerland is simple and easy if you observe a few points for shipping:
The following documents must be enclosed with the shipment:
- Commercial invoice or Proforma invoice*
- Shipping documents / movement certificate in five copies*
*The number of documents may vary depending on the parcel delivery service.
If your company already has a contract with a parcel delivery service, you can prepare all the required documents using the corresponding shipping software.
Commercial invoice
Each shipment outside of the EU is subject to customs duties. This means that samples must be accompanied by a Proforma invoice.
This must contain the following information:
- full description of all content, e.g. “xxx sample for analysis”
- Value of goods: in order to ensure that the samples do not pay additional duty charges, please enter a goods value upwards of €1 for the whole shipment. This allows the analysis samples to remain free of duty.
- Information on the origin of the goods.
Finally, please sign the Proforma invoice.
Shipping documents
The consignment note contains all the details of the shipment and accompanies the shipment until its delivery to UFAG Laboratorien AG. The consignment note number allows you to track your shipment via Track & Trace.
The consignment note should contain the following information:
- sender information
- full recipient’s information
- telephone number of contact person at UFAG.
Delivery times:
There are different rates with different run times depending on the parcel delivery service used.
Generally, express services in Switzerland deliver in 1-2 days. In the case of standard delivery, parcels can take 3-4 or more days depending on the service provider and selected rate.
Do you have further questions? - Our logistics department will be happy to advise you.